Chronic lymphocytic leukemia (CLL) is a type of blood cancer characterized by the abnormal accumulation of mature B lymphocytes. It's the most prevalent form of leukemia in Western countries, especially among older adults.
Key Factors Influencing Treatment:
- Patient Age and Overall Health: Treatment plans are tailored to individual patients.
- CLL Cell Characteristics: Specific genetic factors can affect treatment options.
Treatment Options:
- Observation: For asymptomatic early-stage CLL.
- Chemo-Immunotherapy: For symptomatic or advanced disease.
- Targeted Therapies: Newer treatments like Bruton's tyrosine kinase inhibitors and Bcl-2 inhibitors.
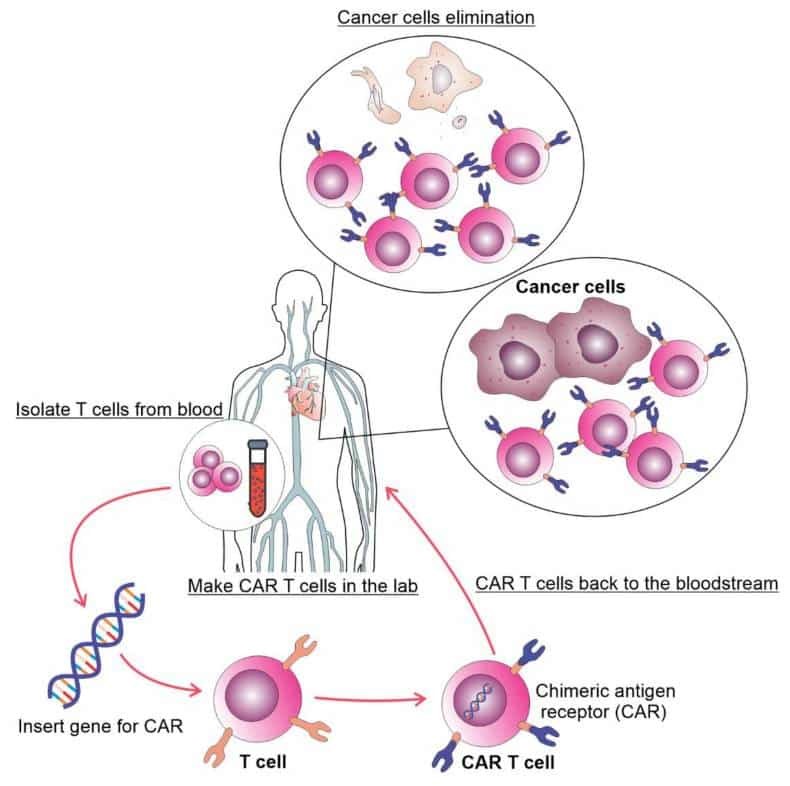
Cell-based immunotherapy has recently become a new and innovative treatment for malignant disorders. One type of immunotherapy called cell-based immunotherapy uses immune cells taken from patients, grown in a lab, and genetically changed to make them better at finding and killing cancer cells (Figure 1). Cancer treatments based on T cells have been made, including lymphocytes that go into tumors, T cells that have had their TCRs changed, and cells that are able to recognize and attack cancer cells.
CAR T cells, a type of engineered T cell therapy, have demonstrated superior efficacy and durability compared to traditional approaches like tumor-infiltrating lymphocytes (TILs) and TCR-modified T cells.
To create CAR T cells, scientists genetically modify T lymphocytes with synthetic receptors that can recognize and eliminate tumor cells expressing specific antigens. These receptors, known as chimeric antigen receptors (CARs), are composed of a T-cell activation domain and a single-chain variable fragment (scFv) of an antibody that binds to tumor-specific antigens.
CAR T Cells: A Multi-Generational Approach
CAR T cells are classified into four generations based on the T-cell activation domain incorporated into their hybrid receptors.
- First-Generation CAR T Cells: These early versions relied solely on the CD3 zeta domain for activation, leading to limited therapeutic efficacy.
- Second-Generation CAR T Cells: By adding one or more costimulatory molecules like CD28, CD40L, or 4-1BB, second-generation CAR T cells demonstrated improved persistence and anti-tumor activity.
- Third-Generation CAR T Cells: Incorporating two or more costimulatory molecules further enhances the immune response of these CAR T cells.
- Fourth-Generation CAR T Cells: Engineered with inducible cytokine expression and a self-withdrawal mechanism, fourth-generation CAR T cells offer a more controlled and targeted approach to cancer therapy.
- Challenges and Innovations in CAR T Cell Therapy
- Despite promising clinical outcomes in certain cancers, CAR T cell therapy is not without its challenges. High medication levels in many patients can lead to reduced T cell quantity and quality, making it difficult to produce and expand autologous CAR T cells. While allogeneic CAR T cells from healthy donors offer potential, they carry the risk of severe graft-versus-host disease (GVHD).
- To address these limitations, researchers have explored the use of allogeneic natural killer (NK) cells as a potential alternative. NK cells are less likely to cause GVHD compared to T cells, making them a promising platform for CAR-modified immune cell therapy. By incorporating CAR constructs, NK cells can be activated and enhanced to target and eliminate cancer cells.
Bristol Myers Squibb's Breyanzi Receives FDA Approval for CLL and SLL
Bristol Myers Squibb announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Breyanzi® (lisocabtagene maraleucel), a groundbreaking CAR T cell therapy targeting CD19, a protein found on certain cancer cells.
Breyanzi is indicated for adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have received at least two prior lines of therapy. It joins Bruton tyrosine kinase (BTK) inhibitors and B-cell lymphoma-2 (BCL-2) inhibitors as treatment options for these conditions.
The FDA's accelerated approval was based on the promising response rate and duration of response observed in clinical trials. However, further confirmation and elucidation of clinical benefits are required in subsequent studies to secure full approval for this specific indication.
Breyanzi is administered through a single infusion after a complex therapeutic procedure. This innovative therapy offers new hope for patients with relapsed or refractory CLL or SLL.
Breyanzi: A Single-Dose CAR T Cell Therapy
Breyanzi consists of a single infusion containing 90 to 110 million viable CAR-positive T cells. For detailed information on Breyanzi, including boxed warnings for Cytokine Release Syndrome (CRS), neurologic toxicities, and secondary hematological malignancies, please refer to the Important Safety Information section.
A Breakthrough for CLL and SLL Patients
"CAR T cell therapies represent a revolutionary treatment option for patients with certain types of blood cancer," stated Bryan Campbell, Senior Vice President and Head of Commercial for Cell Therapy at Bristol Myers Squibb. Despite challenges in developing alternative CAR T cell therapies for relapsed or refractory CLL and SLL, Breyanzi offers a much-needed and innovative treatment choice for these patients.
Expanding Access to CAR T Cell Therapies
By approving Breyanzi as the first CAR T therapy for relapsed or refractory CLL and SLL, the FDA is paving the way for greater access to this transformative treatment. This approval also opens the door for exploring the potential of CAR T cell therapies for a wider range of B-cell malignancies, addressing a critical unmet need in oncology.
.
Challenges in Treating Relapsed or Refractory CLL and SLL
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are common types of B-cell lymphoma. While targeted therapies like BTK and BCL-2 inhibitors have shown promise, many patients eventually experience relapse or develop resistance to these treatments.
For patients who have failed two or more lines of therapy, there is currently no standard treatment approach. This often leads to limited treatment options and unfavorable outcomes, including a lack of durable responses.
TRANSCEND CLL 004: A Phase 1/2 Study of Breyanzi
The TRANSCEND CLL 004 study was a groundbreaking Phase 1/2 clinical trial evaluating the efficacy of Breyanzi, a CAR T cell therapy, in patients with relapsed or refractory CLL or SLL. As the first-of-its-kind, open-label, single-arm study conducted at multiple centers, TRANSCEND CLL 004 demonstrated promising results.
Breyanzi Offers a New Era for Relapsed CLL and SLL
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are typically considered incurable, with limited treatment options for patients who experience relapse or resistance. However, the FDA's approval of Breyanzi represents a significant advancement in the treatment landscape.
Dr. Tanya Siddiqi, Associate Professor in the Division of Lymphoma at City of Hope National Medical Center, emphasized the historical association between complete responses and improved long-term outcomes in CLL and SLL. Breyanzi's approval for patients who have failed BTKi and BCL2i therapy marks a paradigm shift, moving away from continuous sequential treatments towards a personalized T-cell-based approach.
This new approach has the potential to overcome drug resistance and provide patients with complete and durable remissions, offering hope for a more favorable prognosis.
Safety Profile of Breyanzi
The TRANSCEND CLL 004 study included 89 patients treated with Breyanzi. While cytokine release syndrome (CRS) and neurologic events (NEs) were common, most cases were of low severity.
- CRS: 83% of patients experienced CRS of any grade, with 9% experiencing Grade 3 CRS. No Grade 4/5 CRS events were reported.
- NEs: 46% of patients experienced NEs of any grade, with 20% experiencing Grade 3 NEs. One case of Grade 4 NE was documented, and no Grade 5 NEs were reported.
A New Hope for Patients with Relapsed CLL and SLL
Dr. Brian Koffman, a physician, CLL patient, and executive at CLL Society, emphasized the limited treatment options available for patients with relapsed or refractory CLL or SLL. The FDA's approval of Breyanzi as the first CAR T cell therapy for these conditions offers new hope for patients.
With a single infusion, Breyanzi has the potential to provide long-lasting responses in patients with relapsed or refractory CLL or SLL. We extend our gratitude to the patients, their families, and the researchers who contributed to the development of this groundbreaking therapy.
Bristol Myers Squibb is committed to providing comprehensive support to patients and caregivers. Their services include:
- Access to Medicines: Ensuring patients have access to Breyanzi and other vital treatments.
- Cell Therapy 360: A digital platform offering information, updates, and resources to enhance the patient and physician experience.
TRANSCEND CLL 004: A Phase 1/2 Study of Breyanzi
The TRANSCEND CLL 004 study (NCT03331198) is a single-arm, open-label, multicenter clinical trial investigating the efficacy of Breyanzi in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
Study Design:
- Phase 1: Focused on determining the safe and recommended dose of Breyanzi for the subsequent Phase 2.
- Phase 2: Evaluates Breyanzi at the recommended dose from Phase 1.
Primary Objective:
- To assess the percentage of patients achieving a complete response, as defined by the 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria.
This study provides valuable insights into the safety and efficacy of Breyanzi for patients with relapsed or refractory CLL or SLL.
Improved Text: Breyanzi: A Personalized CAR T Cell Therapy for B-Cell Lymphomas
What is Breyanzi?
Breyanzi is a revolutionary cell therapy called CAR T cell therapy. It's designed to target and eliminate cancer cells in adults with specific types of B-cell lymphoma.
How Does Breyanzi Work?
Breyanzi utilizes a patient's own T cells, genetically engineered to recognize and attack cancer cells expressing the CD19 protein. These modified T cells, called CAR T cells, are then infused back into the patient to fight the cancer. The 4-1BB costimulatory domain helps the CAR T cells grow and persist longer in the body.
Approved Uses:
- Relapsed or Refractory Large B-Cell Lymphoma (LBCL): Breyanzi is approved in the US, Japan, and Europe for the treatment of LBCL that has either:
- Relapsed within 12 months of first-line treatment
- Not responded to or relapsed after first-line treatment
- Occurred in patients ineligible for stem cell transplant
- Progressed after two or more lines of therapy
- Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL): Recently, Breyanzi received accelerated approval in the US for CLL/SLL patients who have:
- Failed at least two prior treatments, including BTK and BCL-2 inhibitors
- Relapsed or non-responsive disease
Important Notes:
- Breyanzi is not approved for primary central nervous system lymphoma.
- The CLL/SLL approval is based on initial results and requires further confirmation of clinical benefit in ongoing studies.
Clinical Development:
Bristol Myers Squibb is actively exploring Breyanzi's potential in clinical trials for other lymphoma types.
For More Information:
- Visit clinicaltrials.gov to learn about ongoing Breyanzi studies.
- Consult the full prescribing information for details on treatment process, potential side effects, and limitations of use.




