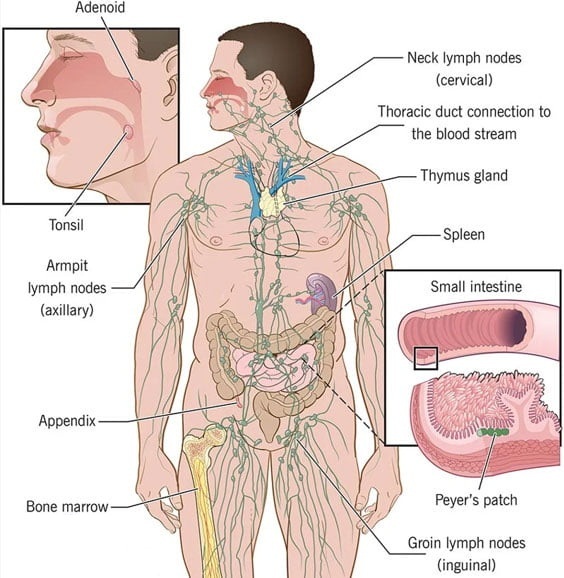
читать далее
Рост медицинского туризма в Китае

China has become a leading global destination for medical tourism, attracting patients from all corners of the world. With world-class healthcare facilities, state-of-the-art technologies, and highly skilled medical professionals, China offers an array of medical treatments ranging from cancer therapies to advanced cosmetic surgery. In this guide, we’ll explore the top medical tourism destinations in China, detailing their specialized treatments, renowned hospitals, and essential tips for international patients.