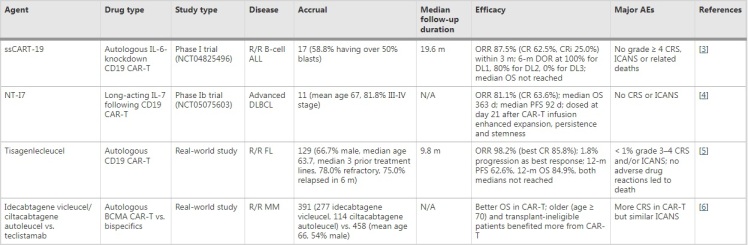
Brain and spinal cord cancers, particularly Diffuse Midline Gliomas (DMGs), are among the most aggressive and fatal types of cancers, especially in children and young adults. These cancers, including the H3K27M-mutant variant, typically progress rapidly and are usually fatal within a year of diagnosis. Standard treatments, such as chemotherapy and radiation, offer only temporary relief and fail to significantly alter the disease’s course. In recent years, CAR T-Cell therapy, an innovative form of immunothera.